Acute myeloid leukemia (AML) is the 2nd most frequent developing hematologic malignancy with deep heterogeneity, but unfortunately the majority of patients relapse even after allogeneic hematopoietic stem cell transplantation (HSCT) in complete remission (CR). We have developed allogeneic HSCT from familial mismatched/haploidentical donors (FMT) in adult patients with a single disease entity of AML lacking of both HLA-identical siblings and well-matched unrelated donors (WMUD) from the mid 1990s. Previously, several pilot studies of FMT performed with various total body irradiation (TBI)-based conditioning regimens (2~12 gray) with or without post-transplant cyclophosphamide (PT-Cy) as a graft-versus-host disease (GvHD) prophylaxis were reported. Here, we demonstrate about the encouraging outcomes of FMT using unmanipulated peripheral blood donor stem cells (PBSCs) and de-escalated dose of TBI (4~8 gray) containing conditioning regimen without T-cell depletion manner.
Although PT-Cy as a specific GvHD prophylaxis regimen is increasingly used for both adult and pediatric FMT, our group investigated the clinical outcomes in adult patients with de novo AML who received full haplotype mismatched HSCT after TBI containing preparation regimen consisted of fractionated TBI (total 4 gray for elderly and 8 gray for <60 years old), fludarabine (150 mg/m 2/day), busulfex (6.4mg/kg/day), and anti-thymocyte globulin (ATG). For GvHD prophylaxis, rabbit ATG (thymoglobulin, Genzyme, 1.25mg/kg/day for 4 days at D-4 to D-1) and a combination of tacrolimus and short-term methotrexate was used. G-CSF mobilized unmanipulated PBSCs were used with a median CD34+ dose of 6.5 x 10 6/kg (1~25.6).
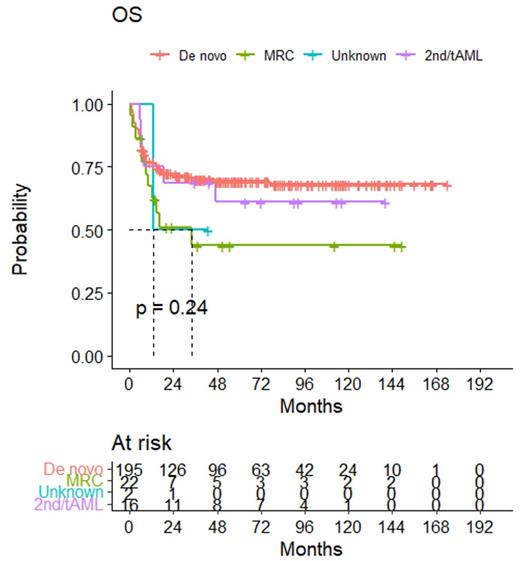
From 2008 to the end of 2022, total 429 adult patients were enrolled, and data demonstrated that our trial of intermediate-dose of ATG and reduced toxicity of TBI-containing conditioning regimen to reduce graft-failure even for high levels of donor-specific antibody titer, early transplant-related mortality, critical cytomegalovirus (CMV) disease, and emerging post-transplant lymphoproliferative disease with rapid immune recovery has shown very successful outcomes. Briefly, the median absolute neutrophil count recovery was 12 days (10~26) and the platelet of 14 days (5~38). There was no either primary or secondary graft-failures at all. With a median follow-up of 36.5 months (2~242) for survivors, overall survival rate (OS) and relapse-free survival (RFS) for patients in any CR (n=338) and relapse/refractory status (n=91) were 62.9% vs. 34.3% and 59.5% vs. 32.2%, respectively. Although there was no significant difference in the subgroup analysis, de novo and young aged patients in any CR showed the best OS 73% (Figure 1). Cumulative incidences of acute GvHD and chronic GvHD were 66% (59-73), 70% (63-76), respectively. The incidences of moderate to severe acute GvHD and chronic GvHD were 16.1%, 30.5%, respectively. By univariate and multivariate analyses, patient's age <54 (p=0.043), pre-HSCT CR1 (p <0.001), absence of mod to severe chronic GvHD (p <0.001), and use of post-transplant iron-chelating agent (p=0.001) were the statistically significant in the study. The GvHD-free and relapse-free survival rate in CR1 was 62.4% for younger patients (age <54) and 47.9% for older patients (p=ns). The non-relapse mortality rate was 27.2% for all enrolled patients and 16% for CR patients. The rapidity of immune recovery was also compared with our old control cohort when we performed T-cell depletion using the CliniMACS system, and it showed a significant reconstitution of CD4+ cells (>300/ul) at 3 months post-FMT compared to the much delayed T-cell depleted FMT. Of note, CMV DNAemia was relatively higher (76.9%) than reported in other studies, likely due to the use of ATG and the endemic region of our country.
Therefore, we suggest that the strategic reduction of fractionated TBI 4~8 gray-based conditioning with unmanipulated PBSCs graft without PT-Cy seems feasible with favorable outcomes for adult patients with de novo AML undergoing FMT in CR, especially even in a certain subgroup of elderly patients with AML.
Disclosures
Kim:LG Chem: Consultancy; Meiji Pharm Co.: Consultancy; Astellas: Consultancy, Honoraria; Boryung Pharm Co.: Consultancy; Janssen: Consultancy; VigenCell: Consultancy; Pfizer: Consultancy; Ingenium: Consultancy; Sanofi Genzyme: Consultancy; AIMS Bioscience: Consultancy; Takeda: Consultancy; Amgen: Consultancy; SL VaxiGen: Consultancy; Novartis: Consultancy, Other: Participation on a Data Safety Monitoring Board or Advisory Board; Handok: Consultancy, Honoraria, Other: Participation on a Data Safety Monitoring Board or Advisory Board; Daiichi Sankyo: Consultancy; BMS&Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety Monitoring Board or Advisory Board; AML-Hub: Consultancy, Other; Abbvie: Consultancy, Honoraria, Other: Participation on a Data Safety Monitoring Board or Advisory Board; BL & H: Other: Grant.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal